Image source: Getty Images.
Since last summer, the market has distributed indiscriminate beatings across the biotechnology industry, and companies without approved products to sell have been hardest hit. The iShares Biotechnology ETF (IBB -0.99%), which tracks hundreds of biotech stocks, has rebounded from low points seen earlier this summer, but it's still 25% lower than where it stood a year ago.
Plenty of biotech stocks remain on sale, you just have to know where to look. Cellular metabolism specialist Agios Pharmaceuticals (AGIO 1.38%) has three candidates under development with its deep-pocketed partner, Celgene (CELG), and a wholly owned orphan drug nearing the finish line.
Shares of another Celgene partner, Juno Therapeutics (JUNO), dove after a temporary issue delayed a clinical trial earlier this month. The issue has been resolved, but the stock hasn't recovered. A third Celgene partner, bluebird bio (BLUE -1.07%) has recovered from low points visited earlier this summer but is still extremely cheap given its pipeline's potential.
It's important to remember these companies have no products to sell, and their stock prices hinge on clinical data. Misfortune could quickly result in heavy losses, but after adjusting for the risk of failure, all three still look like bargains.
1. Agios Pharmaceuticals: a smart partner
Agios entered a discovery deal about six years ago with blood cancer powerhouse Celgene that could help it fund development of its own rare metabolic disease candidates. Over the past couple of years, Celgene picked up various rights to three Agios compounds that target mutant forms of IDH2 and IDH1, two metabolic enzymes commonly found in brain, blood, and other cancer cell types.
Celgene has exclusive worldwide rights to develop and commercialize IDH2-targeting candidate AG-221. The agreement effectively absolves Agios of further risk with the candidate, but it also limits its share of potential sales to $120 million in milestone payments and undisclosed royalties. Celgene also picked up rights to develop and commercialize IDH1-targeting candidate AG-120 outside of the U.S., and it agreed to a down-the-middle cost and profit split for AG-881, which targets both type 1 and 2 IDH mutant enzymes.
These later deals leave Agios more exposed to development and commercial risks, but its slice of any potential profits are also much larger. Hints of success for one of these programs could be around the corner. Furthest along the development path is AG-120 with a phase 3 trial, designed to support an application for treatment of acute myeloid leukemia, slated to begin later this year.

Image source: Getty Images.
I'd say all three Celgene partnered candidates justify the company's $1.71 billion market cap, but including its wholly owned metabolic disease program makes this biotech stock a bargain.
Candidate AG-348 activates normal and mutated pyruvate kinase enzyme, limiting red blood cell destruction and other potentially lethal symptoms of pyruvate kinase deficiency. There are approximately 15,000 to 20,000 people in the U.S. with this inherited disease, which qualifies the candidate as an orphan drug that's subject to reimbursement of a big chunk of development expenses, if approved.
The candidate is making strides in the right direction. In June, Agios announced some promising early data for AG-348 from 18 patients in a phase 2 study intended to enroll 75. Nine of 18 patients treated with either 50mg or 300mg of AG-348 exhibited circulating hemoglobin increases of at least one gram per deciliter (g/dl). For comparison, normal hemoglobin levels for adult males top out around 17 g/dl. It's early, but if these interim results hold steady, a study to support AG-348's application to treat pyruvate kinase deficiency could be next.
With one Celgene-backed candidate ready for a registrational phase 3 trial, a wholly owned orphan candidate probably headed for phase 3, and about $313 million in cash to keep their development humming along, Agios is on sale.
2. bluebird bio: more data, please
Bluebird bio is also discovering and developing cancer therapies in partnership with Celgene, but its wholly owned LentiGlobin cellular therapy for treatment of inherited blood disorders, beta-thalassemia and sickle-cell disease, have gained the most attention. The two diseases affect about 288,000 and 25 million people worldwide, respectively, and bluebird might have a genetic cure for most of them.
Biotech gains or losses following data readouts aren't uncommon, but I can't remember the last time I saw a stock so easily swayed by premature results from so few patients. The stock rose to insane heights based on an early readout suggesting it was a miracle cure for all beta thalassemia patients, but it came crashing down when it revealed two patients with the most severe form of the disease required a single blood transfusion, and a third required multiple transfusions.
Both diseases can be treated with donor stem cell transplants, but the procedure is incredibly dangerous because of graft-vs.-host related issues. Bluebird's LentiGlobin program involves removing a patient's own stem cells, then using a virus to infect those cells with functional hemoglobin genes. Transplants with the modified cells appear to provide a long-term "cure" on par with donor stem cell transplants, but without the lethal side effects.
I think the market has forgotten that regulators are concerned with efficacy and safety. LentiGlobin might not be an absolute cure for every patient, but its combined advantages over existing treatments are enormous. If results seen thus far remain consistent in larger studies, LentiGlobin's applications should be a slam-dunk. With a market cap of $2.11 billion and $553 million in cash and securities at the end of March, this biotech stock is hanging on the clearance rack.
3. Juno Therapeutics: stop and go
Juno Therapeutics is rapidly developing targeted cancer therapies that involve modifying the immune system's T-cells outside the body. Celgene is so intrigued by the technology's potential, it bought a 9.8% equity stake in Juno, and more recently exercised its option to develop and potentially sell candidates from Juno's programs targeting CD19, a protein often found on cancer cells, outside of North America and China.
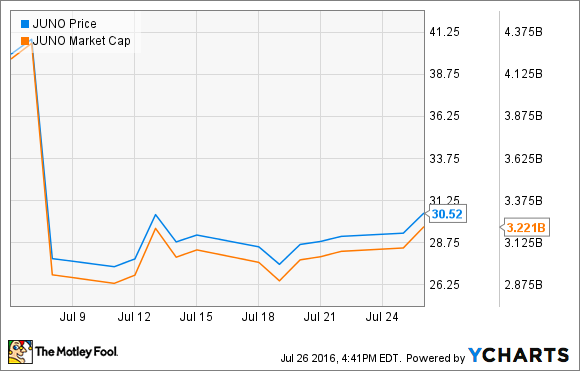
The lead candidate in that program, JCAR015, is in a phase 2 study treating acute lymphoblastic leukemia patients who have relapsed after multiple therapies. On July 7, the FDA slammed the brakes on all JCAR015 trials following two patient deaths, and the stock fell about 30% overnight.
As it announced the clinical hold, Juno had already singled out the culprit: one of two chemo drugs used to pre-condition patients before receiving Juno's candidate. Just five days later, the FDA allowed the trial to continue, but without the aforementioned chemo drug.
The regulator acted much faster than it had to, which I view as a sign of encouragement. The market doesn't agree, and at recent prices, Juno stock is still 25% lower than it was before announcing the hold. With a market cap of $3.2 billion, eight candidates in clinical stages, and $927 in cash and securities to fund their development, Juno Therapeutics stock has been marked down to a bargain-bin price we might not see again.