Clinical trial highlights took a back seat last week to the stream of blockbuster deals in the health care sector. Still, there was a big winner and a big loser -- even if they flew relatively under the radar. Spectrum Pharmaceuticals (SPPI) was, in my opinion, the clear winner, having made headway toward improving pre-transplant chemotherapy for multiple myeloma patients.
The week's biggest catastrophe goes to Cytokinetics (CYTK -1.86%), with another mid-stage muscle modulator failure testing its investors' threshold for pain.
The win
Spectrum Pharmaceuticals announced some success last week with a recently purchased formulation of an old chemotherapy agent. The Captisol-enabled Melphalan that it acquired from Ligand Pharmaceuticals (LGND -5.44%) hit its primary endpoint in a multiple myeloma trial. It was just a phase 2 trial, but Spectrum plans on taking the data to the FDA next quarter.
This is great news for both companies. Ligand is still eligible to receive more than $50 million in milestone payments. That's just a bit more than company's total revenue over the past twelve months. If approved, potential royalties in the 15% to 25% range from CE Melphalan sales would also go a long way toward pleasing investors.
Spectrum's stock popped more than 8% following the news last Wednesday, but the good times didn't last long. It quickly gave up those gains, ending last week flat. For a company with just four commercial products, the muted response to the clinical win would seem on the surface to be a little counter-intuitive.
No surprise
Multiple myeloma patients about to receive stem cell transplants are typically treated with Melphalan first. The idea is to destroy diseased cells first so that the new cells get a running start.
Melphalan is typically administered with propylene glycol, a solvent with known renal and cardiac side effects that limits dosage. The trial found that Captisol-fused Melphalan was safer and less toxic, but that was hardly surprising. At least half a dozen FDA-approved drugs employ Ligand's Captisol technology, including Bristol-Myers Squibb's Abilify and Amgen's (AMGN 1.11%) Kyprolis.
It seems that the announcement's lack of efficacy data upset the market. The secondary objectives of the trial included measuring rates of response, myeloablation, and engraftment. Without any figures to offer, Spectrum's promise that "additional analyses are currently under way" was less than satisfying.
The catastrophe
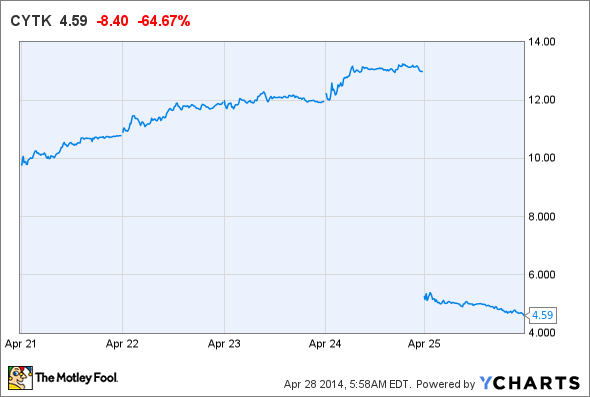
Cytokinetics fell so hard last Friday, you can almost hear a "thud" just looking at this chart.
One of the company's lead drug candidates, tirasemtiv, flubbed a mid-stage trial in amyotrophic lateral sclerosis (also known as ALS or Lou Gehrig's disease.) Patients receiving the skeletal muscle activator showed some improvement versus the placebo group, but not enough to be considered significant.
The data given offers optimists nothing to hold on to. With an estimated enrollment of 680 patients, it's unlikely that a few outliers skewed the data. Mixed results from tests of secondary objectives aren't encouraging either.
Repeat offender
Tirasemtiv isn't the only troubled program at Cytokinetics. Last September, Amgen reported a lack of success with an acute heart failure candidate licensed from Cytokinetics. Omecamtiv mecarbil was supposed to give the heart a boost, but it failed to significantly improve shortness of breath in heart failure patients.
Amgen did its best to interpret the results from this first of two trials in a positive light. There was a trend that suggested that a higher dosage led to stronger response, and patients receiving the highest dose showed a significant response.
Amgen is still running a second trial with omecamtiv mecarbil. The main difference is the delivery method: the trial that failed was administered as an infusion, while the ongoing trial is delivered orally.
Following the failure, Amgen stated that it would consider combined results from both trials before deciding to continue the program. Topline results are expected by the end of the year. If the data isn't much better than the first trial, you might hear another "thud" from Cytokinetics' stock chart.