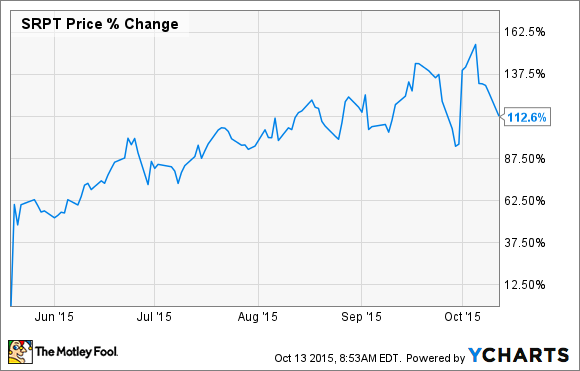
Shares of Sarepta Therapeutics (SRPT 0.51%) have been on fire ever since the U.S. Food and Drug Administration announced earlier this year that it was finally willing to review the biotech's regulatory filing for eteplirsen, its experimental Duchenne muscular dystrophy (DMD) drug:
With the drug's Prescription Drug User Fee Act (PDUFA) action date scheduled for Feb. 26, 2016, now is as good a time as any to consider the stock's potential risks and rewards ahead of this pivotal catalyst.
Upside potential
As the chart above shows, Sarepta has more than doubled in value in a few short months. So, right off the bat, it's not unreasonable to assume that eteplirsen's approval is already baked into the company's present valuation.
But let's take a deeper look. According to six analysts surveyed by S&P Capital IQ, Sarepta's 2019 revenue consensus currently stands at $390 million if eteplirsen is approved this coming February. The low revenue estimate for this same time period sits at $207 million, and the high is at $549 million.
If we use consensus as our starting point, Sarepta's shares would seem to be trading at around four times 2019 projected revenue right now.
Is that expensive? For an orphan drug specialist like Sarepta, the answer is definitely no. Many of its rare-disease peers, for example, trade around nine to 10 times their 2019 revenue projections. That suggests that Sarepta could double again if the FDA gives eteplirsen the green light.
Downside risk
The problem, though, is that eteplirsen's regulatory review is anything but a slam dunk. While I personally believe the FDA will request that Sarepta complete its ongoing late-stage study prior to an approval, my Foolish colleague Dr. Brian Orelli recently gave a compelling argument for why the agency may let the drug onto the market -- despite some glaring deficiencies in its regulatory application.
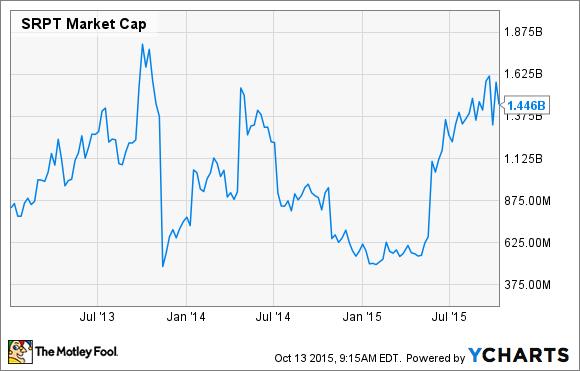
But given that we can't really know what's going to happen with eteplirsen during this review cycle, investors should most certainly weigh the downside risks. The good news is that an inspection of the recent history of the company's market cap seems to provide us with at least some insight into the risks that may be lurking in the shadows:
SRPT Market Cap data by YCharts.
What this chart shows is that Sarepta's market cap has dipped twice below the $625 million mark following regulatory setbacks for eteplirsen. If we use this as our baseline, Sarepta's downside could be as high as 56% if the FDA rejects eteplirsen next year.
Is Sarepta worth the risk?
Based on this quick and dirty analysis, Sarepta's upside potential appears to still outweigh its downside risk. But I would argue that there are also some hidden dangers that may tip the balance toward more of an even draw.
Sarepta is applying for an approval for eteplirsen based on a small midstage study. That means an approval would probably be conditional, pending the outcome of a larger confirmatory trial. So, in essence, Sarepta's shareholders are facing not one, but two binary events at this point (eteplirsen's forthcoming regulatory review in 2016 and the outcome of a late-stage trial expected in 2018).
All told, Sarepta has been a great stock this year, and it certainly has the tools to continue this trend into 2016. But I think the significant risks involved make this stock fit only for seasoned biotech investors who are realistic about the FDA's unpredictable nature when it comes to approving new drugs.