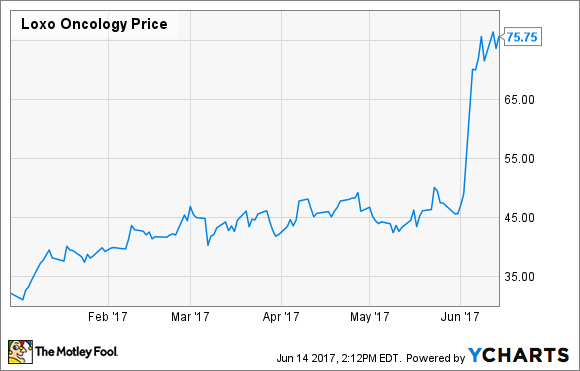
Usually, when a biotechnology company announces it's raising more money from investors, the news causes shares to sell off. However, shares in Loxo Oncology (LOXO) rallied despite the announcement of a shelf offering for an undisclosed number of shares. The news follows prior share sales to boost its balance sheet, including a 5 million-share offering in January of 4,450,500 shares at $31.00 per share that raised about $138 million, gross of fees.
The new share offering follows a busy few weeks at Loxo Oncology that was highlighted by the release of trial data for its lead cancer drug, larotrectinib, at the annual American Society of Clinical Oncology (ASCO) meeting earlier this month.

IMAGE SOURCE: GETTY IMAGES.
Bolstering its balance sheet
Cancer drug trials have a notoriously high failure rate, and given that they're costly to conduct, it's not surprising that biotech companies such as Loxo Oncology raise money after good news sends their share prices higher.
In this case, high response rates in various cancer patients with a specific genetic abnormality sent shares up more than 50%, and with trials still ongoing, plans for an FDA filing for larotrectinib in place, and the potential cost of commercialization on the horizon, management can't be blamed for taking advantage of the run-up to increase its financial wiggle room.
The company hasn't said how many shares it's selling, but an offering similar to January's isn't out of the question. Currently, it has cash, cash equivalents, and investments of $244.3 million exiting March, and as of April, that cushion had management saying it had enough backing to fund operations into the middle of 2019.
Delivering on promises
Larotrectinib is a TRK-fusion drug that takes a unique approach to cancer treatment. Unlike traditional cancer drugs that the FDA evaluates based on their efficacy and safety in patients with cancer originating in a specific location of their body, larotrectinib targets patients with TRK-fusion genetic abnormalities, regardless of a cancer's location.
TRK signaling is important to neuronal synapses, memory development, and maintenance and the protection of neurons, but its activity normally declines after birth. Sometimes, however, it can fuse together with other genes, causing cancer growth and proliferation. These fusions have been associated with various cancers, including lung cancer, head and neck cancer, melanoma, colorectal cancer, and breast cancer.
To explore the use of larotrectinib as a TRK inhibitor that prevents this from happening, Loxo Oncology is being studied across these various tumor types, and at ASCO, the company reported a 76% objective response rate in patients to it, including 64% who partially responded and 12% who were completely responded to the drug.
The company hopes to use this efficacy data as the basis for a filing for FDA approval later this year, or early in 2018. If regulators eventually greenlight larotrectinib, management estimates its addressable market is between 1,500 and 5,000 late-stage eligible patients in America, plus a similar proportion of patients in large European countries and Japan.
Eyes on the future
Loxo Oncology will use money raised from the shelf registration to fund larotrectinib's ongoing development, its FDA filing, the development of other next-generation TRK-fusion drugs in its pipeline, and pre-commercialization activities.
An eventual FDA go-ahead, however, isn't guaranteed. Undeniably, the data so far is compelling, but the company won't file for larotrectinib's approval until it has results from an independent review of its patient data that confirms the drug's efficacy.
Currently, results are based on a radiology review by people who are affiliated with the study's investigators. A final independent review may back up the data, or it could raise questions, depending on the findings. Only time will tell how this shakes out.
Nevertheless, the prospect of winning FDA approval of larotrectinib based on genetic factors, rather than cancer location, is incredibly exciting, and it could be very rewarding for investors. Just remember before buying shares that a lot can -- and often does -- go wrong with clinical cancer trials, and therefore, this is a high-risk stock best suited to aggressive investors who can withstand a significant stumble.