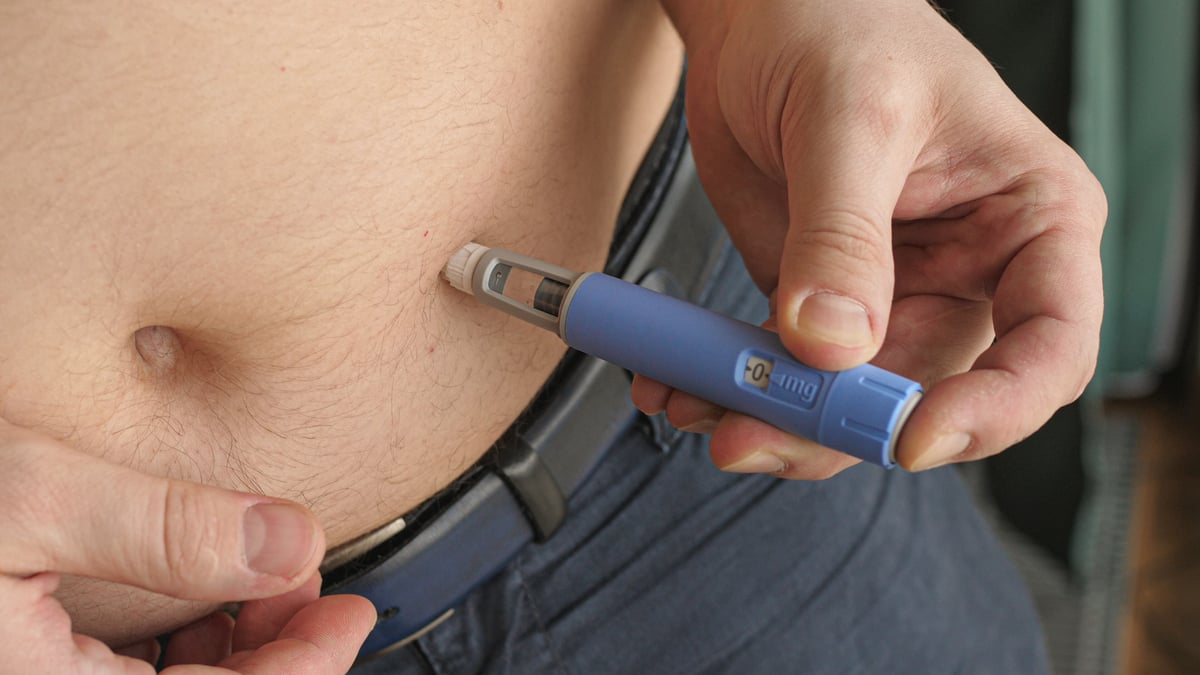
The Food and Drug Administration is investigating two deaths that occurred after patients were injected with Eli Lilly's (LLY +1.80%) antipsychotic Zyprexa Relprevv.
The drug is the long-acting version of the oral version of Zyprexa, which is taken daily. Zyprexa Relprevv is injected every two to four weeks.
Lilly had trouble getting Zyprexa Relprevv approved, and when it did, the FDA slapped a black box warning recommending that patients be observed by a health care professional for at least three hours after each injection because high levels of the drug can cause sedation and delirium.
The two patients died three to four days after receiving the dose, well after the observation period. Both had high levels of the active ingredient in their blood.
Not the end of the world
We're still in the investigation phase. At this point, there appears to only be a correlation, but causation hasn't been established just yet. Even if Zyprexa Relprevv is pulled from the market because the FDA is worried that the drug is released faster than it's supposed to, it won't be a major issue for Eli Lilly.
The company doesn't even bother breaking out sales of Zyprexa Relprevv in its earnings report. Bloomberg reported that a Lilly representative said Zyprexa Relprevv sales accounted for less than $60 million last year. That's out of $22.6 billion, or about 0.2% of revenue. Losing the drug would hardly be noticed, certainly nothing like Eli Lilly experienced when the oral version, which was selling $4.6 billion per year, started seeing generic competition.
Johnson & Johnson (JNJ +0.75%) is the leader for the long-acting antipsychotic, selling $1.4 billion worth of Risperdal Consta and $800 million worth of Invega Sustenna last year. Alkermes (ALKS 2.30%) also benefits from a royalty on both drugs. It developed the extended-release technology in Risperdal Consta, and Alkermes acquired the royalty rights to Invega Sustenna when it bought Elan's (ELN +0.00%) drug technology division.
Those sales are pretty impressive considering that the oral version of Risperdal is available as a generic. Doctors clearly see an advantage in having schizophrenic patients not taking daily oral medications, where compliance isn't always as high as doctors would like. Since they have to be injected by a health-care professional, prescribing the injected medication also allows psychiatrists more frequent contact with the patient and better drug monitoring.