It isn't often when I have the pleasure of reporting positive news when it comes to a serious disease, but two new approvals from the Food and Drug Administration over the past week should give new hope to suffers of HIV, or people who are at high-risk of contracting the disease.
According to the Centers for Disease Control and Prevention, 50,000 new cases of HIV are reported each year, with some 15,500 people dying from the disease in 2010. Furthermore, of what is estimated to be 1,148,200 people in the U.S. living with HIV infection as of the end of 2009, roughly 200,000, or 18%, had no clue they had the disease.
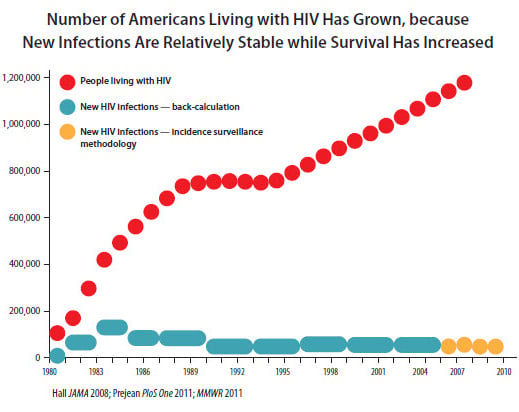
Source: Centers for Disease Control and Prevention.
HIV infection is unfortunately very efficient at damaging its host's autoimmune system, so increased public awareness about the dangers of HIV contraction, as well as improved medications over the past two decades, have played an increasingly crucial role in attempting to lessen incidence rates of the disease and severity of the symptoms.
The good news is that two new approvals from the FDA over the past week are helping to change both the preventative aspects of contracting the disease, as well as improve patients' quality of life for those who do have the disease.
More choices equal better care
On Friday, we received exciting news from Alere (NYSE: ALR) that the FDA had approved its pre-market application to market Alere Determine, an HIV-1/HIV-2 diagnostic test. Not all HIV infections are the same, and this diagnostic test will help rapidly determine what type of HIV antibody a patient may be infected with, resulting in a faster and customizable treatment plan. In addition, Alere's point-of-care test can detect HIV-1 p24 antigens which appear in a patient before the appearance of HIV-1/HIV-2 antibodies. In short, Alere's point-of-care diagnostic is now one of the most accurate early detection tests for HIV-1 or HIV-2 around!
The good news didn't stop there, though. Yesterday, following the closing bell, the FDA announced that it had approved Tivicay (previously dolutegravir), an oral drug used in combination with other antiretroviral drugs, for the treatment of HIV-1. Developed by ViiV Healthcare -- which is owned in large part by GlaxoSmithKline (GSK +0.39%), but also by Pfizer (PFE 1.26%) and Shionogi Pharmaceuticals --Tivicay delivered impressive results in all of its late-stage trials, including one such trial that demonstrated a fully suppressed viral load (i.e., an undetectable level of the disease) in 63% of patients after 24 weeks.
What's unique about Tivicay is that it has the potential to treat an even broader audience than four-in-one pill Stribild, which Gilead Sciences (GILD +0.57%) developed to treat HIV-1 infection in treatment-naive patients and approved in August of last year. Tivicay, on the other hand, was approved to treat HIV-1 infection in patients who have been treated with HIV medications previously and in treatment-naive patients. Furthermore, whereas Stribild is approved to treat adults only, Tivicay proved safe in treating children in late-stage trials and, as such, was granted approval to treat children as young as 12, who weigh at least 40 kg, and who haven't taken an HIV drug previously with the same mechanism of action.
Is Stribild yesterday's news?
Following Gilead's impressive run over the past year on the heels of its Stribild approval, and what seems like a no-brainer approval later this year for sofosbuvir, the company's highly effective oral hepatitis-C treatment, some investors might interpret last night's approval of Tivicay as a sign of worry. In actuality, it's far from it.
Tivicay is going to really open up potential treatment options for those who've taken Stribild, or some other HIV medication, and either failed to respond or were forced to discontinue taking the drug they were on because of adverse side effects. It's also a big boost for those aged 12 to 17 who previously had limited options available to them and are at high risk of developing, or already have, HIV-1 infection.
On an overall basis, Stribild still looks like it'll remain the go-to once-daily medication for physicians in treating HIV-1 infection in treatment-naive patients. In two double-blind late-stage trials, Stribild delivered a fully suppressed viral load of 88% to 90%, which -- although Tivicay was never put in a head-to-head against Stribild -- should be more than enough for physicians to stick with Gilead's still relatively new drug.
Peak sales estimates for Tivicay are roughly $900 million, according to a poll conducted by Thomson Reuters. By contrast, Stribild's peak sales estimates come in nearly as high as $5 billion thanks to its overwhelming effectiveness in trials. Furthermore, the great news for Gilead shareholders is that Stribild, which is a completely in-house drug, will slowly replace Atripla, a three-in-one HIV drug that combines one compound from Gilead, one from Johnson & Johnson (JNJ 0.72%), and one from Bristol-Myers Squibb. Cutting these other two drugmakers out of the equation will lead to considerably beefier margins for Gilead.
The state of HIV infection treatment moving forward
Make no mistake about it: A new diagnostic test and new HIV-1 oral medication that will broaden the treatment range to teenagers and those who've been previously treated but proved unresponsive or unable to continue is fantastic news -- but the work isn't over yet. Until these drugmakers can achieve 100% viral load suppression, the battle against HIV will rage on.
Just last month I examined an intriguing new vaccine being developed by ViiV Healthcare and J&J that combines one compound from each company into a once-monthly shot. In a study involving 40 HIV-negative patients, the once-monthly vaccine proved potent enough to control or slow progression of the disease, with its effect being seen up to four months after the final dosing. If this treatment proves as effective as Stribild in larger studies, we could be looking at a revolutionary shift away from pills and back to shots if it means being taken once a month or even less often.
For now, though, I believe we'll continue to see Gilead's Stribild dominate among HIV-naive patients, with Glaxo & Pfizer's Tivicay picking up some scraps and Alere's point-of-care diagnostics seeing big gains all the way around. However, with HIV research remaining such a hot-button and dynamic topic, I'd recommend keeping one eye peeled with regard to the latest research on this disease.









