What happened
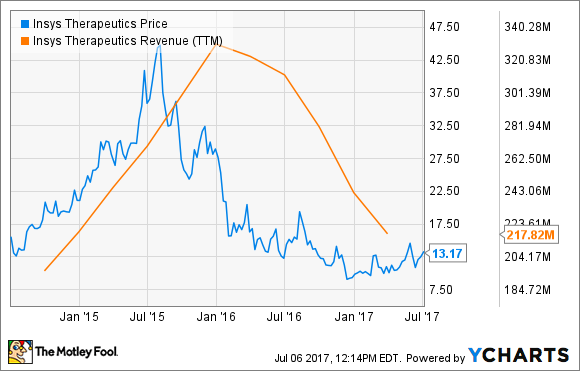
June was a crazy month for Insys Therapeutics (INSY) investors.
Shares of the medical marijuana company tumbled in the first half of the month after the FDA asked competitor Endo International to remove its opioid pain medicine from the market. Shares rallied in the back half of the month after FDA comments led people to believe opioids that are used to treat cancer pain, including Insys Therapeutics' Subsys, would avoid a similar fate.
When all was said and done, Insys Therapeutics lost 11% of its value in the month, according to S&P Global Market Intelligence.

Image source: Getty Images.
So what
Insys Therapeutics is close to launching Syndros, a reformulation of the long-standing marijuana drug Marinol, but until that launch is complete, it relies entirely on Subsys for its sales.
In the past year, demand for Subsys has dried up following reports of widespread off-label use. The potent fentanyl spray is only approved for use in breakthrough cancer pain, but up to 80% of its prescriptions were reportedly for other unapproved indications.
Because opioid abuse has reached epidemic proportions, regulators are increasingly scrutinizing their use, and that's increasing concern that the FDA will request more opioid medications are taken off the market. Scrutiny is also leading to doctors writing fewer prescription for these drugs. Unsurprisingly, this has taken a big toll on Insys Therapeutics sales and, correspondingly, its share price.
While there's still reason to worry that Subsys could fall into the FDA's cross-hairs, a little concern abated after Janet Woodcock, the FDA director of the Center for Drug Evaluation and Research, told NPR, "The overall societal consequences of the abuse of these drugs is tremendous and terrible, and more definitely needs to be done. At the same time, of course the FDA-we have to keep pain medicines available, say, for people who have terminal cancer and so forth."
FDA commissioner Scott Gottlieb also weighed in on the opioid crisis last month with a public statement that focused on the use of abuse-deterrent opioids for chronic pain, rather than for the treatment of cancer pain. Altogether, these comments suggest that FDA regulators aren't focusing too much attention on cancer pain drugs like Subsys.
Now what
The company's potential role in the off-label prescribing of Subsys has caused multiple reshufflings of its C-suite, but it may (finally) have found a permanent CEO. In April, former Purdue Pharmaceuticals executive Saeed Motahari was given the company's reins, and since then, he's been bringing in a team of fresh faces.
This new management team has a lot of work to do. They'll need to resolve any outstanding investigations into Insys Therapeutics marketing of Subsys, execute a successful launch of Syndros, and kick-start their research and development program, including efforts to create new medicines based on cannabidiol, a non-psychoactive marijuana cannabinoid.
Ultimately, it's anyone's guess if Motahari will succeed in overcoming the company's challenges, but Insys Therapeutics shares have already fallen pretty far, and the potential associated with developing new medical marijuana drugs may make this stock worth watching.