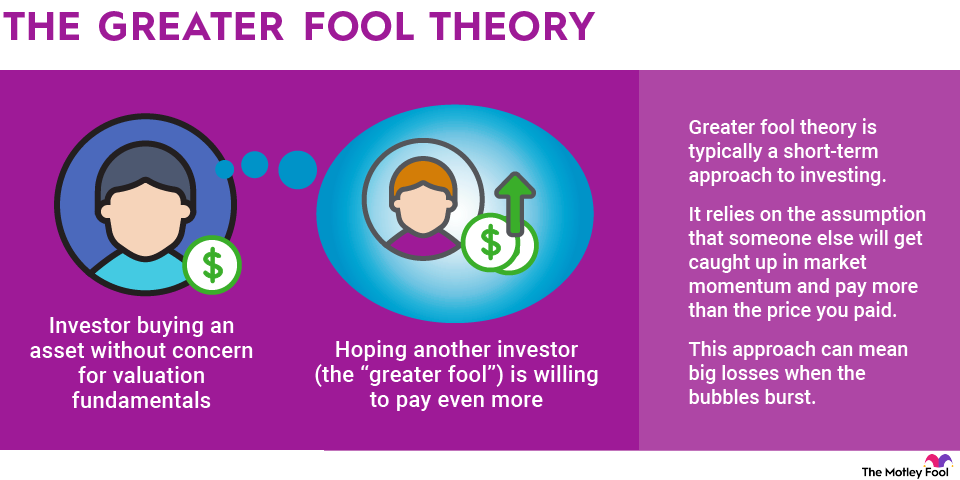
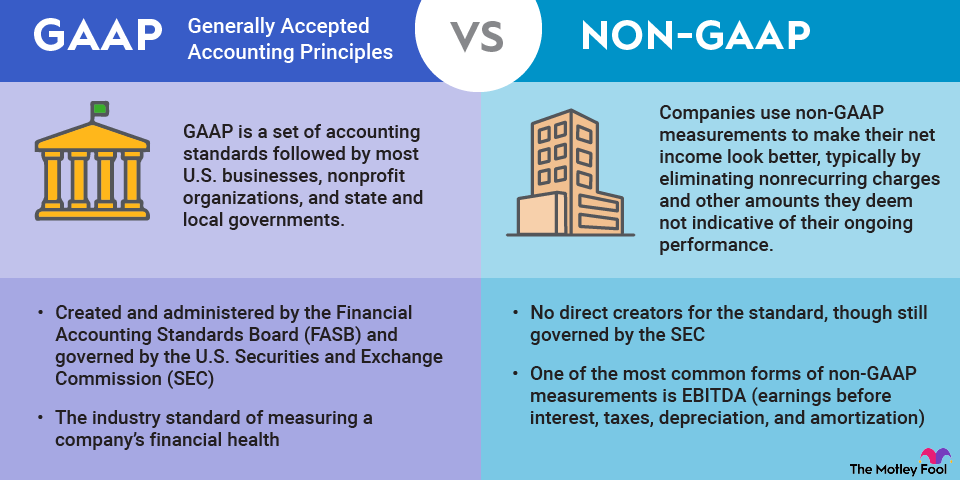
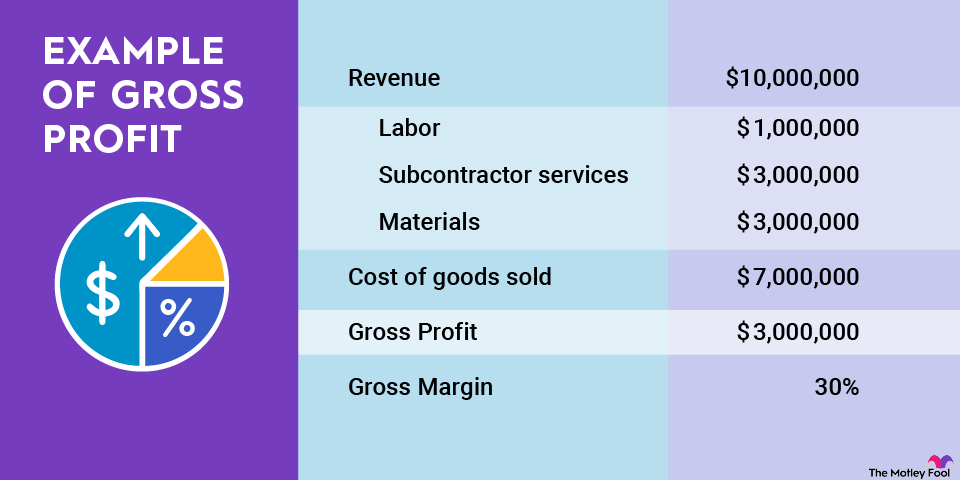
Why do generic drugs matter to investors?
Drug patents usually last about 20 years from the date of filing. Because of the extensive time required for testing and clinical trials, a drug might spend significantly less time on the market before its patent expires. After that, competitors can begin making generics, which can push down prices for a brand-name drug and cut into margins.
For these reasons, investing in companies that specialize in generic drugs can be appealing. These drugs are cheap to produce by comparison and get approved quickly, since the maker of the brand-name version already did most of the hard work.
Manufacturers of generic drugs operate on much thinner margins, but can produce a much larger catalog of drugs due to the relative ease of the approval process. Brand-name drugs may command larger margins, but they cost significantly more to develop and produce. When exclusivity expires, drug makers often face a patent cliff, or a steep drop in revenue as generic manufacturers introduce cheaper versions.