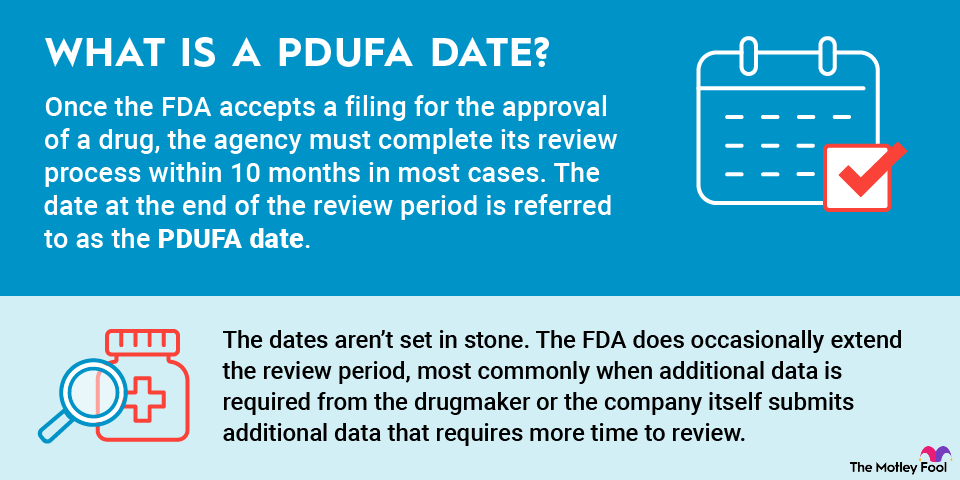
History of the PDUFA date
Prior to 1992, the FDA didn't have to make approval decisions within a specified period. This caused frustration for drugmakers, along with physicians and patients hoping for new treatments.
The FDA, too, knew that it had a problem with speed. The agency also needed additional money to pay for extra staff to expedite the review process for regulatory filings.
The PDUFA served as a compromise between the pharmaceutical industry and the U.S. government. Although drugmakers had previously been reluctant to pay increased fees to the FDA, they went along with the idea in exchange for faster drug approval times. Through the years, the pharmaceutical industry began to champion PDUFA reauthorization and the process for establishing PDUFA dates.
Related investing topics