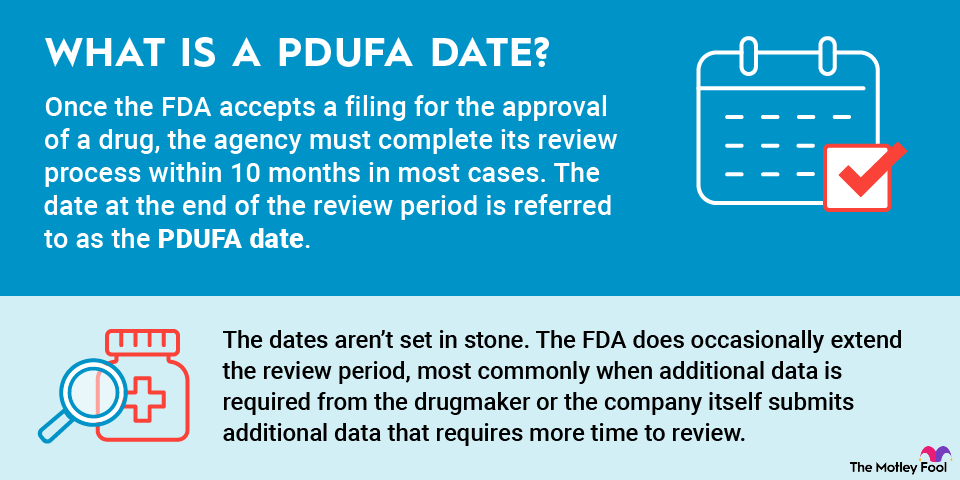
What is a PDUFA date?
Once the FDA accepts a filing for the approval of a drug, the agency must complete its review process within 10 months in most cases. The date at the end of the review period is referred to as the PDUFA date.
In some instances, the FDA grants Priority Review status to the regulatory filing for a drug. This designation is given to therapies that, if approved, would significantly improve the safety or effectiveness of the treatment, diagnosis, or prevention of serious diseases. When the FDA grants Priority Review for a drug, the PDUFA data is six months from the acceptance of the submission.